
| Cadmium | |
| Electrons per shell | 2,8,18,18,2 |
| Electron Configuration | [Kr] 4d10 5s2  |
| Ground state | 1S0 |
| Atomic Volume | 13.1 cm3 mol-1 |
| Electronegativity | 1.69 |
| Magnetic ordering | No data |
| Mass magnetic susceptibility | -2.3 x 10-9 |
| Molar magnetic susceptibility | -2.59 x 10-10 |
| Speed of sound | 2310 m s-1 |
 Thermal Properties Thermal Properties |
|
| Enthalpy of Atomization | 113 kJ mol-1 @25°C |
| Enthalpy of Fusion | 6.11 kJ mol-1 |
| Enthalpy of Vaporisation | 100 kJ mol-1 |
| Heat Capacity | 26.020 J mol-1 K-1 |
| Thermal Conductivity | 96.6 W m-1 K-1 |
| Thermal Expansion | 30.8 μm m-1 K-1 |
 Hardness Hardness |
|
| Brinell hardness | 203 MPa |
| Mohs hardness | 2.0 |
 Elastic Properties Elastic Properties |
|
| Bulk modulus | 42 GPa |
| Poisson ratio | 0.30 |
| Shear modulus | 19 GPa |
| Young's modulus | 50 GPa |
 Electrical Properties Electrical Properties |
|
| Electrical resistivity | 7 x 10-8 Ω m |
| Electrical conductivity | 0.138 106/cm Ω |
 Chemical Properties Chemical Properties |
|
| Electrochemical Equivalent | 2.097 g Ah-1 |
| Electron Work Function | 4.22 eV |
| Valence Electron Potential (-eV) | 30 |
| Ionization Potential | |
| 8.993 | |
| 16.908 | |
| 37.48 | |
| Incompatibilities | Strong oxidizers, ammonium nitrate (NH4NO3), hydrazoic acid (HN3), tellurium, zinc, elemental sulfur, selenium. |
 Flammability Flammability |
|
 Energy Levels Energy Levels |
|
| 23.1736 KeV | |
| 22.9841 KeV | |
| 26.0955 KeV | |
| 26.6398 KeV | |
| 26.056 KeV | |
| 3.13373 KeV | |
| 3.12691 KeV | |
| 3.31657 KeV | |
| 3.52812 KeV | |
| 3.3993 KeV | |
| 3.3661 KeV | |
| 3.71686 KeV | |
| 3.9522 KeV | |
| 2.956 KeV | |
| 2.7644 KeV | |
 Atomic Radii Atomic Radii |
|
| 155 pm | |
| 161 pm | |
| 148 pm | |
| 158 pm | |
| No data. | |
| 151 pm | |
 Oxidation States Oxidation States |
|
| Cd+2 | |
| Cd+1 | |
 Ionisation Energies (kJ mol-1) Ionisation Energies (kJ mol-1)  |
|
| 867.6 | |
| 1631 | |
| 3616 | |
| 5300 | |
| 7000 | |
| 9100 | |
| 11100 | |
| 14100 | |
| 16400 | |
| 18800 | |
 Vapour Pressure Vapour Pressure |
||||||
| P (Pa) | 1 | 10 | 100 | 1K | 10K | 100K |
| T (K) | 530 | 583 | 654 | 745 | 867 | 1040 |
 Crystal Structure Crystal Structure |
|
| Hexagon close packed | |
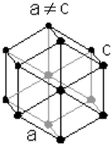 |
a = 297.94 pm |
| b = 297.94 pm | |
| c = 297.94 pm | |
| α = 90° | |
| β = 90° | |
| γ = 120° | |